Abstract

Background: Mutations in isocitrate dehydrogenase 1 (mIDH1) occur in approximately 6-10% of patients (pts) with AML. Ivosidenib (IVO), a potent, oral, targeted inhibitor of mIDH1, is FDA approved as monotherapy for the treatment of relapsed/refractory AML and newly-diagnosed AML (ND-AML) in adults >75 years of age or with comorbidities that preclude the use of intensive chemotherapy. In addition, the combination of IVO with azacitidine (AZA) was recently FDA approved in older or unfit AML pts with mIDH1 as a result of the global Phase 3 AGILE trial (NCT03173248). Compared to placebo (PBO) + AZA, the combination of IVO + AZA extended event-free survival (EFS; hazard ratio 0.33, 95% CI [0.16 - 0.69]; P = 0.002), overall survival (OS; median 24.0 months vs. 7.9 months; hazard ratio 0.44; 95% CI [0.27 - 0.73]; P = 0.001), and increased the rates of complete remission or complete remission with partial hematologic recovery (CR/CRh; 52.8% vs. 17.6%). To date, biomarker analysis has shown that IVO+AZA is associated with deep and durable clearance of IDH1m clones with improved clinical responses and EFS/OS in several pt subgroups, including those with mutations in receptor tyrosine kinase (RTK) pathway genes, a subgroup refractory to IVO monotherapy.
Aim: To characterize clonal evolution and relapse mechanisms in pts with mIDH1 ND-AML treated with IVO+AZA.
Methods: Patients received oral IVO + AZA per study protocol. Next-generation DNA sequencing (DNA-seq) on baseline and longitudinal samples was performed on bone marrow aspirates (BMA), bone marrow mononuclear cells (BMMCs), or peripheral blood mononuclear cells (PBMCs) depending on sample availability using the ACE Extended Cancer Panel (Personalis, Inc); limit of detection (LOD) 2% variant allele frequency (VAF). This analysis was focused on variants in 95 AML-associated genes with known or likely oncogenic potential.
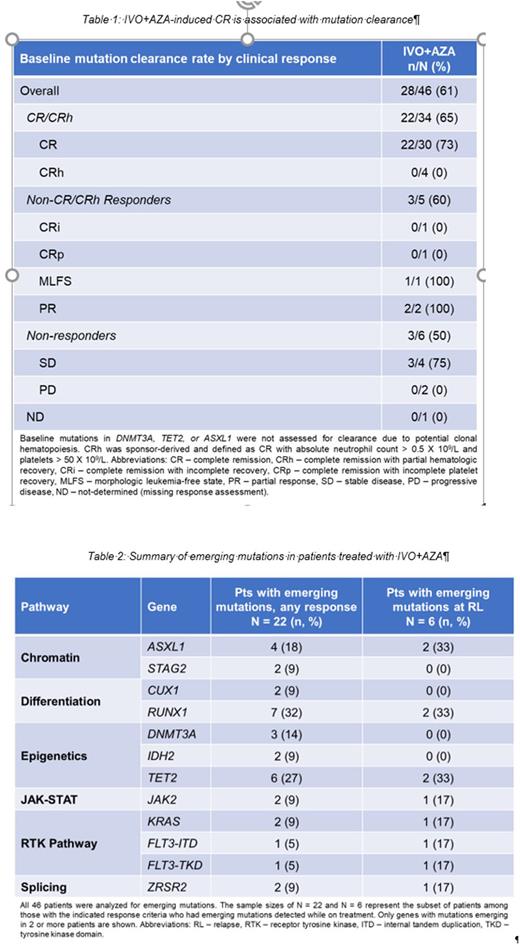
Results: Among 72 pts enrolled on the IVO+AZA arm, 46 had both baseline and longitudinal DNA-seq data available, with a median follow-up of 366 days on study (range 16 - 1210). The median number of baseline mutations was 3 (range 1 - 10). Suppression of all baseline non-DTA (DNMT3A / TET2 / ASXL1) mutations to below the LOD ("mutation clearance") was observed in 28 patients, including 22/34 (65%) pts who achieved CR/CRh [Table 1].
To identify potential mechanisms of acquired resistance, on-treatment samples were analyzed for the emergence of mutations not detected at baseline. Overall, emerging mutations were identified in 22/46 (48%) pts (median 2 emerging mutations; range 1-6). 2/46 (4%) pts had emerging mutations in IDH2, but discontinued treatment due to adverse events and stable disease, respectively. No second-site IDH1 mutations were observed across any response category.
Longitudinal samples were available for 8/10 (80%) pts who discontinued treatment due to relapse (RL) following an objective response (4 CR, 2 CRh, 1 CRi, 1 MLFS). Emerging mutations were detected in 6/8 (75%) of RL pts [Table 2]. Genes with emerging mutations recurrently associated with RL included ASXL1, FLT3, RUNX1, and TET2 (2 pts each). The IDH1 mutation was not detected at RL in 6/8 (75%) pts. Among the two RLs that occurred in the absence of emerging mutations, one was associated with expansion of a baseline FLT3-ITD subclone, and one was not associated with any new emerging or expanding mutations. To further evaluate clonal evolution and disease progression of these pts, scDNA-seq is being performed and will be presented.
Conclusion: IVO+AZA combination therapy leads to deep and durable remissions associated with clearance of mIDH1 and baseline co-mutations. To date, no second-site IDH1 mutations have been observed, but mutations in other genes, including IDH2, can emerge and may provide an opportunity for relapse. Relapses were observed in a subset of responders and were generally associated with expansion or emergence of high-risk mutations occurring independently of mIDH1. Further study is needed to better characterize mechanisms of RL to IVO+AZA.
Disclosures
Döhner:Syndax: Consultancy, Honoraria; Kronos Bio: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Berlin-Chemie: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; GEMoaB: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Servier: Other: Travel, Accommodations, Expenses; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees. Marchione:Servier Pharmaceuticals: Current Employment. Choe:Servier Pharmaceuticals: Current Employment. Montesinos:Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; Takeda: Consultancy, Research Funding; Astellas: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Menarini/Stemline: Consultancy, Research Funding; Otsuka: Consultancy; Kura Oncology: Consultancy; Incyte: Consultancy; Ryvu: Consultancy; Nerviano: Consultancy; Beigene: Consultancy. Recher:Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, BMS-Celgene, Otsuka, Astellas, Daiichi-Sankyo, Macrogenics, Roche, Takeda, Servier, Pfizer: Other: Advisory role; Pfizer: Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie, Amgen, Novartis, BMS-Celgene, Jazz Pharmaceuticals, Agios, MaatPharma, Astellas, Roche, Iqvia, Daiichi-Sankyo: Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Wang:AbbVie: Membership on an entity's Board of Directors or advisory committees. Cerchione:Sierra Oncology: Honoraria, Speakers Bureau; Karyopharm: Honoraria, Speakers Bureau; Jazz Pharmaceutical: Honoraria, Speakers Bureau; Bristol Myers Squibb: Honoraria, Speakers Bureau; AbbVie: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; GlaxoSmithKline: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Astellas: Honoraria, Speakers Bureau; Beigene: Honoraria, Speakers Bureau; Servier: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Glycomimetics: Consultancy; Menarini-Stemline: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau. Heuser:Eurocept: Honoraria; Janssen: Honoraria; Takeda: Honoraria; BMS: Consultancy; Kura Oncology: Consultancy; PinotBio: Consultancy; Tolremo: Consultancy; Astellas: Research Funding; Bayer Pharma AG: Research Funding; BergenBio: Research Funding; Loxo Oncology: Research Funding; Agios: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Calado:Alexion: Honoraria; Novartis: Honoraria; Agios: Membership on an entity's Board of Directors or advisory committees. Schuh:AbbVie: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Teva Pharmaceutical Industries: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Phebra: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlycoMimetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Yeh:Amgen: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Chugai Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Janssen Biotech: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda Oncology: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. De La Fuente:Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Daiichi Sankyo: Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau. Hui:Servier Pharmaceuticals: Current Employment. Patel:Servier Pharmaceuticals: Current Employment. Gianolio:Servier Pharmaceuticals: Current Employment. Daigle:Servier Pharmaceuticals: Current Employment. DiNardo:LOXO: Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Honoraria; Cleave: Research Funding; Novartis: Honoraria; Takeda: Honoraria; Foghorn: Honoraria, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Research Funding; Astex: Research Funding; ImmuneOnc: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Astellas: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Forma: Research Funding; Jazz: Honoraria; Gilead: Honoraria. De Botton:BMS: Consultancy, Honoraria, Speakers Bureau; Abbvie: Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Servier: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Astellas: Honoraria, Speakers Bureau; GSK: Consultancy; Syndax: Consultancy; Forma: Research Funding; Auron: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.